200,000 Recall (Three Lots) of Concentrated MOTRIN® Infants Drops Original Berry Flavor 1/2 fl oz
The Concentrated MOTRIN® Infants Drops (DYE FREE) Original Berry Flavor 1/2 fl oz is NOT Included in the Recall.
SOURCE McNeil Consumer Healthcare (Johnson & Johnson)
 — McNeil Consumer Healthcare Division of McNEIL-PPC, Inc. (“McNeil”) is voluntarily recalling at the retail level three lots, approximately 200,000 bottles, of Concentrated MOTRIN® Infants’ Drops Original Berry Flavor 1/2 fl oz bottles distributed in the United States (see full product list below). This recall is being conducted with the knowledge of the U.S. Food and Drug Administration (FDA). McNeil is asking retailers to remove the affected lots from store shelves and is asking consumers to stop using and dispose of any product they may have that is included in this recall.
— McNeil Consumer Healthcare Division of McNEIL-PPC, Inc. (“McNeil”) is voluntarily recalling at the retail level three lots, approximately 200,000 bottles, of Concentrated MOTRIN® Infants’ Drops Original Berry Flavor 1/2 fl oz bottles distributed in the United States (see full product list below). This recall is being conducted with the knowledge of the U.S. Food and Drug Administration (FDA). McNeil is asking retailers to remove the affected lots from store shelves and is asking consumers to stop using and dispose of any product they may have that is included in this recall.
After releasing these three lots of Concentrated MOTRIN® Infants’ Drops Original Berry Flavor 1/2 fl oz into the market, tiny plastic particles (approximately 1 mm in size or about the size of a poppy seed) were identified in a different product lot during manufacturing. This lot was not released to the market. It was determined that the particles originated in a shipment from a third party supplier of ibuprofen, the active ingredient in Concentrated MOTRIN® Infants’ Drops Original Berry Flavor 1/2 fl oz. Out of an abundance of caution, McNeil is voluntarily recalling the three lots released to the market made with the same batch of active ingredient. McNeil has worked with the third party to ensure that corrective measures are currently in place and are effective. The potential for adverse medical events related to the reason for this recall is not likely. Concentrated Infants’ MOTRIN® Drops Dye-Free Berry Flavor 1 fl oz is not included in this recall. Children’s or Adult MOTRIN® products are not included in this recall.
If you have any questions or concerns, or would like to inquire about a refund, please call our Consumer Care Center at 1-877-414-7709. Parents and caregivers with any health questions or concerns should contact their healthcare provider and visit www.motrin.com for additional information.
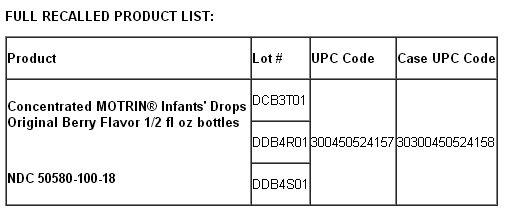
FULL RECALLED PRODUCT LIST:
Adverse events that may be related to the use of this product may be reported to U.S. Food and Drug Administration’s (FDA) MedWatch Adverse Event Reporting Program either online, by regular mail or by fax:
Online: www.fda.gov/medwatch/report.htm
Regular mail: Use postage-paid, pre-addressed Form FDA 3500 available at: www.fda.gov/MedWatch/getforms.htm. Mail to address on the pre-addressed form.
Smokey Barn News (Sponsor/Advertisement)





particularly binaural cartier become desirable amongst sports athletes, organizations, and those operating to their personal advancement to religious notice condition. These include popular all over the worldwide for the meditation and also as A very good furthermore quick technique at bypass all aware mind to function right With all the subconscious. In the last few years They’ve get much more popular.
i got it for my girlfriend as one of her christmas presents and was worried that it might look and feel cheap since i got it for a good price. When i got it i was releaved how nice it looked and im no leather pro but it feels somewhat real to me. not a bad deal at all